![From Pentalene to Dicyclopenta[b,g]naphthalene, or the Change towards Delocalized Structures - García Cuesta - 2006 - ChemPhysChem - Wiley Online Library From Pentalene to Dicyclopenta[b,g]naphthalene, or the Change towards Delocalized Structures - García Cuesta - 2006 - ChemPhysChem - Wiley Online Library](https://chemistry-europe.onlinelibrary.wiley.com/cms/asset/3df9f588-d9f8-4a93-be07-6207082f6c53/mcontent.gif)
From Pentalene to Dicyclopenta[b,g]naphthalene, or the Change towards Delocalized Structures - García Cuesta - 2006 - ChemPhysChem - Wiley Online Library
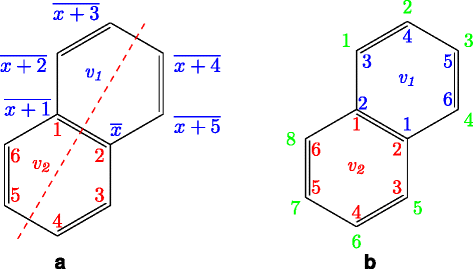
Enumeration method for tree-like chemical compounds with benzene rings and naphthalene rings by breadth-first search order | BMC Bioinformatics | Full Text
Naphthalene aromatic hydrocarbon molecule. Skeletal formula illustration Stock Vector Image & Art - Alamy
Naphthalene aromatic hydrocarbon molecule. Skeletal formula illustration Stock Vector Image & Art - Alamy

Magnetically induced ring currents in naphthalene-fused heteroporphyrinoids - Physical Chemistry Chemical Physics (RSC Publishing)




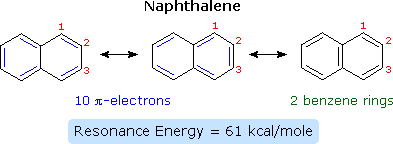






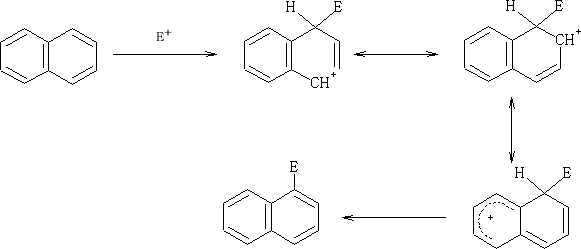

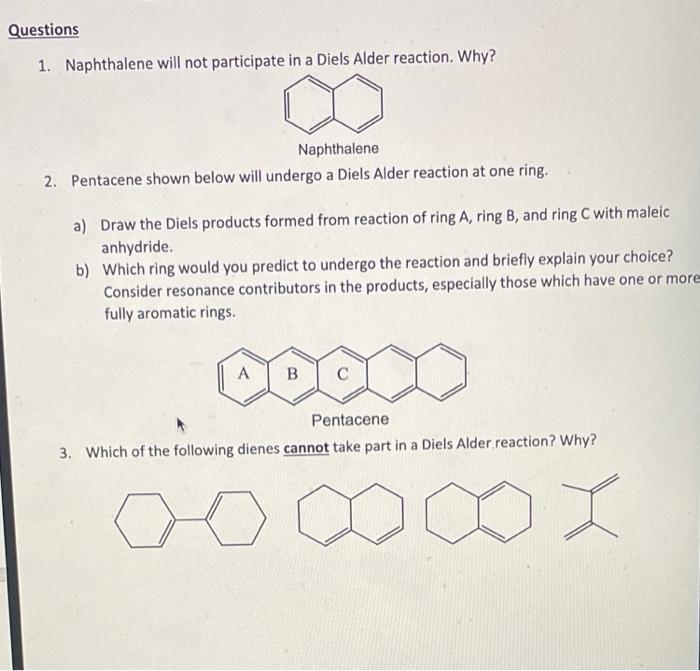

.png?revision=1&size=bestfit&width=260&height=121)